Today’s Chemical: Mercury
What is Mercury? Mercury is an element with an atomic number of 80. While mercury is a metal, it is a liquid at room temperature, which makes it both really cool and really useful. Mercury exists in many different forms, and the form that it’s in makes a big difference: the different forms are used for different things, and their chemistry and toxicity varies greatly. Here are the major forms of mercury and their uses:
- Elemental mercury: Used in thermometers, pressure sensing devices (like barometers), dental fillings, industrial processes, and fluorescent light bulbs.
- Mercury salts: This category includes things like mercury chloride, mercury nitrate, and mercury sulfide. These used to be used as medicines (laxatives, Syphillis treatments), for various industrial processes, paints, and cosmetics, but now they are primarily used as disinfectants and pesticides.
- Organic mercury compounds: Once used as pesticides, but not used for much these days. However, this is the major form found in the environment.
How are people exposed to mercury?
Welcome to Bad Science on the Internet! Here, we highlight some of the crazy and sometime dangerous stuff people post online, and then give you the facts.
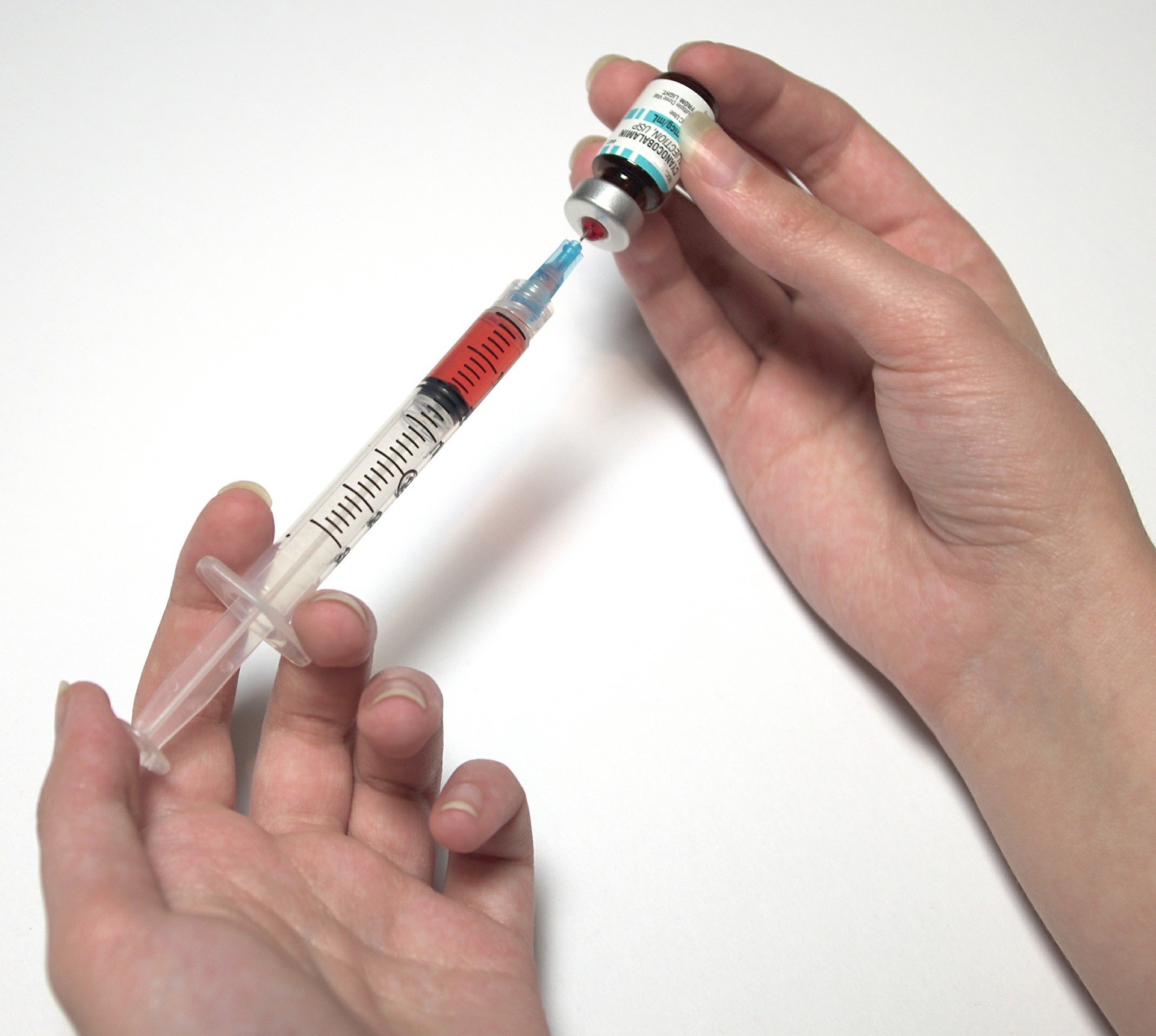
The bad science: A recent article circulating around Facebook claims that the new flu vaccines don’t work and are actually dangerous because of new additives in the vaccines.
What do they claim? The main claims are that the new flu vaccines are not safe and that it’s less risky to skip the vaccine this year and risk the flu.
Are they trying to sell you something? Just the usual anti-vaccine conspiracy theory madness. This article was distributed on Facebook by a site called “Earth We Are One”, but there is no tag line identifying the author or even giving a date. It’s not clear who wrote this or why.
Is any of this true? It is actually amazing how little of this is true. Let’s go point-by-point!
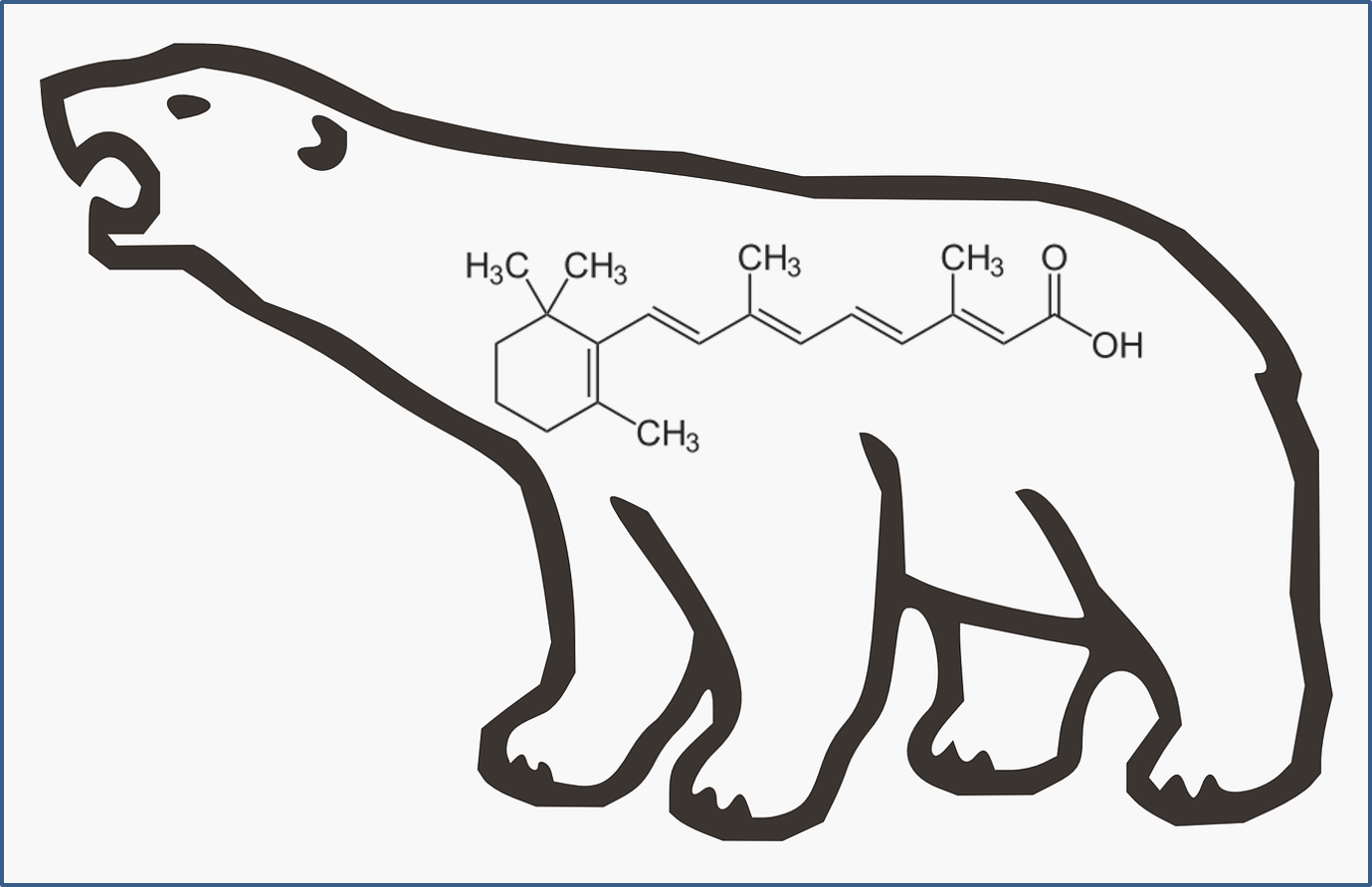
Today’s Chemical: Vitamin A (retinal, retinol, retinyl esters, and retinoic acid)
What is vitamin A and how are people exposed to it?
“Vitamin A” is technically a group of very similar chemicals. The main forms in the body are retinal, retinol, retinyl esters, and retinoic acid. Vitamin A is required for normal development and function in all vertebrates (animals with a backbone) and some non-vertebrates (like worms and bugs). Since animals can’t make vitamin A from scratch, they require dietary sources – either from plants containing carotenes (you’ve probably heard of beta-carotene, but did you know there were alpha and gamma forms too?) or by eating other animals who had already produced vitamin A from a plant source. several forms of vitamin A (tretinoin, isotretinoin, and alitretinoin) are also FDA-approved drugs, used to treat severe acne and some rare skin cancers.
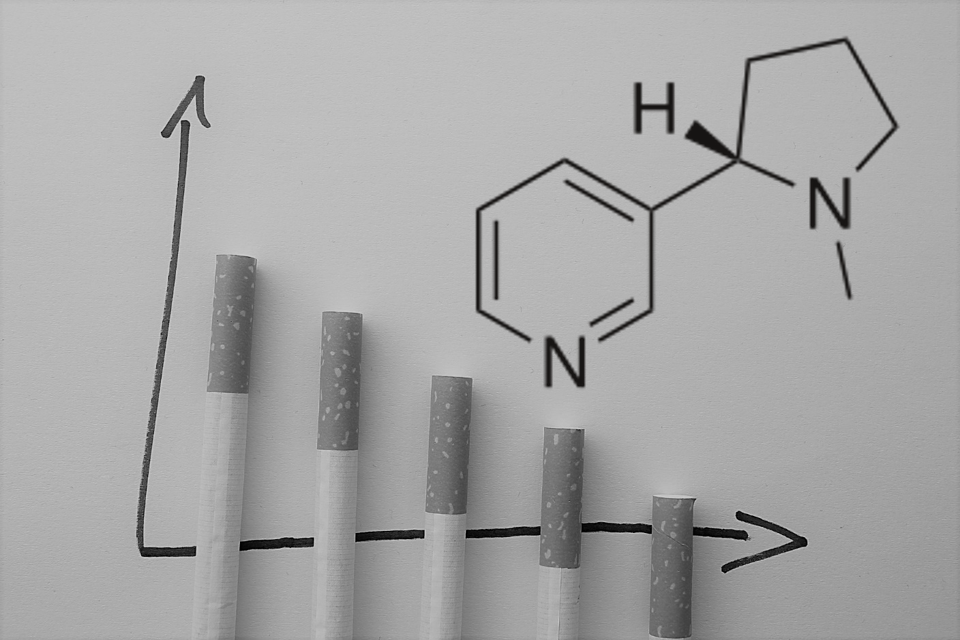
Today’s Chemical: Nicotine

What is Nicotine and how are people exposed to it?
Nicotine is an alkaloid produced naturally by a number of plants in the night shade family, most notably the tobacco plant. People are mainly exposed to nicotine by smoking (cigarettes, cigars, pipes, smokeless tobacco), but also via vaping or e-cigarettes. Some people may be exposed to nicotine or related chemicals when using them as insecticides on crops.
What does Nicotine do?
Plants produce nicotine as an insecticide to keep insects from eating their leaves. Nicotine binds to nicotinic acetylcholine receptors (nAChRs) in the central nervous systems of insects, causing paralysis and death. In mammals, the nAChR receptor subtypes are different, and as a consequence, nicotine binds more weakly and stimulates the nervous system, causing the characteristic addictive “high” that people seek out by smoking.
Nicotine itself (sold as nicotine sulfate) can be used as a pesticide, though it is not currently sold in the U.S. Nicotine derivatives (known as neonicotinoids) are widely used as insecticides across the world. They have come under quite a bit of scrutiny lately because these insecticides are very good at killing bees – and could be one of the contributors to colony collapse disorder.
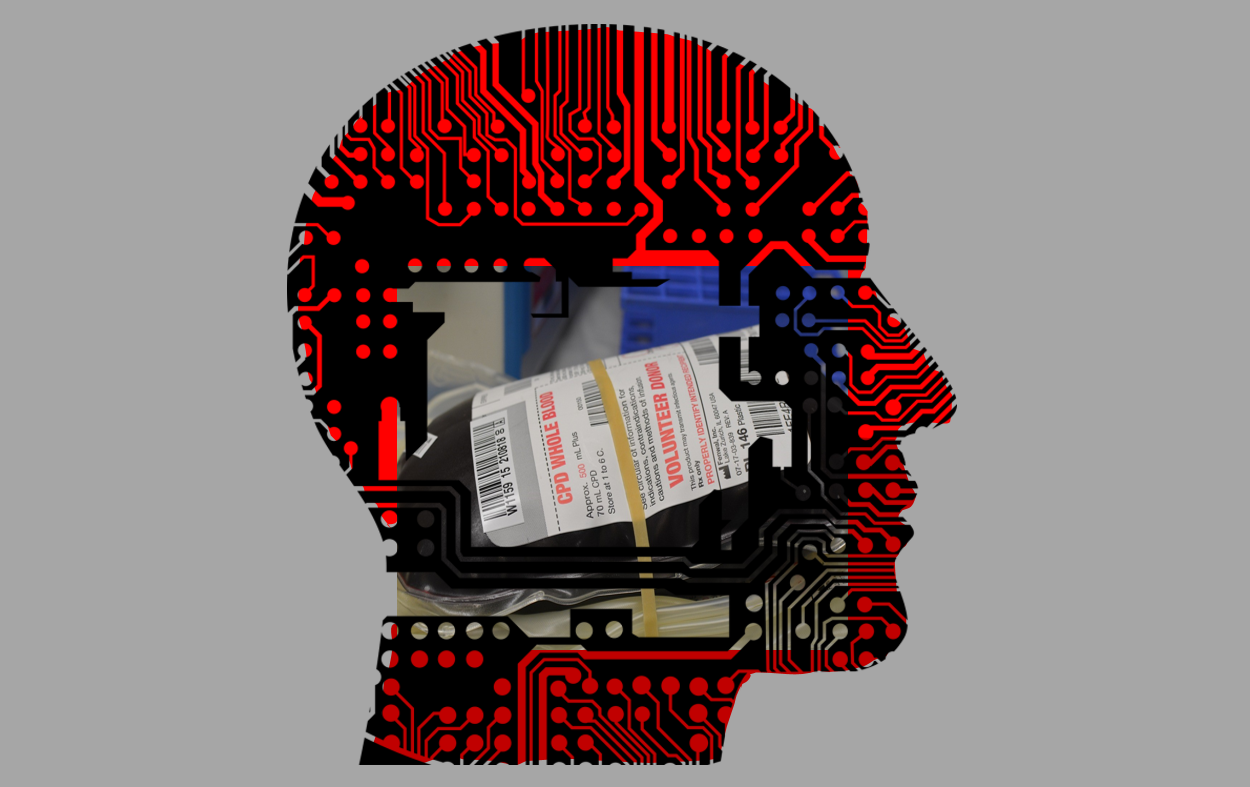
Are “blood boys” like the one on Silicon Valley a real thing, and if so do they work? – D.L. Madison, WI
Yes! They totally are! Well, sort of – here’s the skinny:

The idea of using the blood of younger people to heal or slow the aging process has been around for a while. There has been a recent resurgence in interest for two reasons. First, some intriguing new data has been generated and widely publicized, and second, some folks in Silicon Valley (the place, not the show) may actually be trying it.
The data has come from “heterochronic parabiosis” experiments in mice. Heterochronic parabiosis is the surgical pairing of the circulatory systems of two animals of different ages. Importantly, these experiments are performed in an attempt to understand the role of blood (and the factors it contains) versus the cells in the aging process – not in an attempt to develop a treatment for aging. Studies have shown that the cells in older mice benefit from being exposed to the blood of younger animals. That sounds great! However, there are some caveats. First, these mice are usually surgically connected for weeks to months of time – these effects have never been shown with a single or even multiple infusions – only continuous parabiosis. Second, the younger animals experience negative effects (their cells act like they are older), so it’s not something anyone is likely to sign up for.
Is pink slime dangerous and how can I avoid it? – K.B. Chico, CA
Great question! Here’s the skinny:
What is “pink slime”?
“Pink slime” is a pejorative term for “lean finely textured beef” (LFTB), “finely textured beef” (FTB), or boneless lean beef trimmings (BLBT). It’s added into ground beef as a filler and to reduce the fat content of the final product. To make pink slime, boneless beef trimmings (small leftover pieces of beef) are heated, centrifuged (spun around really fast to remove the fat), frozen and crushed into a paste. To disinfect it, ammonia or citric acid treatment is often used.

The term was coined by a USDA scientist by the name of Gerald Zirnstein. He didn’t like the look of the pink slime (to be fair, almost no one does), and he objected to it being classified as meat (he was overruled by his USDA superiors). Zirnstein may have had a point – this is probably stretching the definition of meat that most of us would accept. Basically, they took some fatty pieces of meat, removed most of the fat and turned what was left into a paste in the process – I guess that’s sorta meat? More like a meat puree? Whatever you call it, there is little doubt that it is made from meat, so we’ll just call it meat and move on.
Using ammonia to disinfect the LFTB has received much of the media attention, partly because it is specifically banned from use in Canada and the European Union (EU). However, Canada and the EU allow similar products which could be described as pink slime. The citric-acid treated LFTB is used in Canada and the EU allows something called “de-sinewed meat” or “red meat paste.” These all sound gross.